This is a trick question. An electron within an atom doesn’t have a velocity. Despite what we are usually taught in grade school, it isn’t a particle zipping in an orbit around the nucleus. An electron is better described as having an orbital rather than an orbit. “Orbital” sounds something like an orbit, but it isn’t.
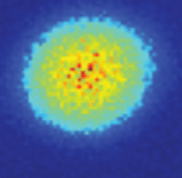

An analogy for an orbital is a cloud. The image to the left is a computer-generated reconstruction of an electron orbital, also called an “electron cloud.”

Sometimes physicists calculate a velocity that corresponds to the amount of energy in the electron cloud. This approach is beyond my understanding. But I’ve read on-line in a couple of places that if the energy of an electron orbital in a hydrogen atom were translated into velocity, it would travel over 2,200 kilometers a second. At this speed, if the electron were to orbit, which it doesn’t, it would travel around the Earth in a little over 18 seconds. So, an electron orbital has a lot of energy, but it doesn’t have a velocity.
