Category: Particle-wave duality
Are electrons waves or particles?
The accompanying video demonstrates how an electron can be both a particle and a wave. (The video has two unfortunate errors in it, which I’ll point out.) The video shows how different kinds of objects, including an electron, act when they speed towards a barrier perforated by two slits. Then, it shows the pattern the objects form on a detection screen after passing through the slits in the barrier. This is the famous Double Slit Experiment. Here’s what’s going on in this video, step by step:
How Particles Act

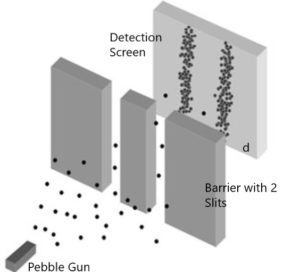
“Particle” shows ordinary particles, let’s say pebbles. Particles are separate individual little things that at any one moment are in a tiny, very localized position. So, individual pebbles shoot through one slit or the other. Then, the pebbles hit the detection screen.
Here’s where the video goes off the rails. It shows a pattern on the detection screen of random dots all over the screen. Your common sense would tell you that the pebbles should form two clumps on the detection screen, one behind each slit. Real experiments show that your common sense is correct. Figure (1) by Fermilab is a more accurate depiction of what the detection screen should look like.
How Ordinary Waves Act
“Wave,” in the video, shows an ordinary wave, let’s say a water wave. The water wave is spread out, so it goes through both slits. On the far side of the barrier, two waves emerge, one from each slit. The two waves interfere with each other. They form the crisscross pattern of ripples that we see if we throw two stones into a pond. This crisscross
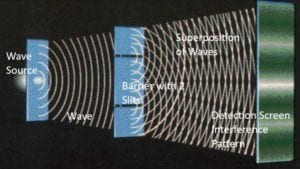
Figure (2) clearly shows how a water wave creates the crisscross “interference pattern” and marks the detection screen with a striped pattern. This is just like in the video. This striping is the signature pattern of waves interacting. When physicists see this pattern, they think “waves.”
To summarize, here are the differences from particle behavior: the wave is spread-out; goes through both slits, not just one or the other; forms two interacting waves on the far side of the barrier; and forms a striped pattern on the detection screen.
How Electrons Act
“Quantum object” shows a subatomic particle, for example, our electron. It doesn’t act at all like an ordinary particle such as a pebble. At first, it acts more like a wave. It’s spread out and goes through both slits. It emerges as two different waves on the far side of the barrier, and these interfere with each other. The two waves form the same crisscross pattern that ordinary waves form.
But upon hitting the detection screen, the wave “collapses.” The electron wave hits the screen in one tiny spot as if it were a particle. The experiment is run over and over. One at a time, electrons flow wave-like through the barrier and collapse at the detection screen, each time hitting one tiny spot, that is, suddenly turning into a particle. Over time, a pattern on the detection screen emerges. It’s the striped pattern—the signature pattern of two waves interacting! Somehow the particles which hit the screen “know” where to land on the detection screen such that over time, they collectively seem to show the influence of the two interacting waves.
The Quantum Wave vs. Ordinary Wave
Even though the electron acts in certain ways like a wave, there are significant differences between the wave of a quantum particle and an ordinary wave like a water wave. The electron type-wave is called a “quantum wave.” An ordinary wave is called a “classical wave.” The mathematical equations which describe the properties of a quantum wave and a classical wave are very different. While quantum waves share some similarities of behavior with classical waves, for example, creating a striped pattern on the detection screen, quantum waves also act significantly differently. Quantum waves and classical waves differ in both their mathematical descriptions and in their behavior.
Here’s a brief listing of differences between a quantum wave and a classical wave (for more detail see the article in this encyclopedia on wave):
- As shown in the video, the quantum wave collapses when it hits the detection screen and lands on it as a particle. This is called the “collapse of the wave function.” An ordinary wave retains its wave nature when it hits the detection screen.
- The amplitude of a quantum wave is proportional to the probability that the quantum particle will be detected in a specific position. In contrast, the amplitude of a classical wave is proportional to the wave’s strength.
- The equation of a quantum wave can include imaginary numbers. These are numbers that include the square root of negative 1. As no number times itself is a negative number, imaginary numbers do not refer to anything that has physical reality. The equations for classical waves do not include imaginary numbers and describe physically real things.
- It is when an electron is in the quantum wave state, rather than in its particle state, that it displays quantum weirdness: superposition (being in more than one place at the same time), entanglement (behaving in an instantaneously correlated manner with an electron as far as across the universe), quantum tunneling (appearing on the other side of a barrier despite having insufficient energy to cross the barrier), and other weirdnesses. Classical waves, of course, do none of these things.
Wave-Particle Duality
*The Transactional Interpretation is explained in lay terms without math in Ruth E. Kastner, Understanding Our Unseen Reality, Solving Quantum Riddles; Imperial College Press, 2015, London.
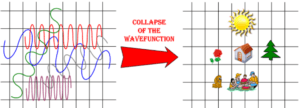
How does the electron enter our physical reality? It interacts with something physical that is made up of lots of particles—a “macroscopic object” like a detection screen. Upon interacting with the screen, it’s suddenly a particle. This is called the “collapse of the wave function.” Since, the electron always becomes a particle as soon as it interacts with a macroscopic object, we can never observe it in its wavy state. We’re like King Midas. He could never feel his daughter’s soft hand because she turns to gold the moment that he touches her. We can never observe the wavy state of an electron because the wave function collapses to a particle when we interact with it sufficiently to perceive it.
Figure (3) depicts Quantumland on the left, the collapse of the wave function, and the resultant objects in everyday spacetime on the right. This depiction is a gross simplification because the collapse from wave to particle does not occur at the scale of entire objects like homes and picnicking families. Instead electrons, quarks, and other quantum particles are continually moving from their wavy states, interacting with others, and collapsing to particles. When they collapse, the entire atom and molecule collapses with them. Then, quantum particles revert to their wavy state, collapse again, and on and on. So, at any one moment, many of the atoms and molecules of an object are in their wavy state and many are collapsed down to particles.
Add an Observer
This brings us to the final part of the accompanying video, “Add an Observer.” This part of the video shows the electron wave approaching the barrier. But this time, there’s a detector at the barrier watching which slit the electron goes through. This could be a human with Superman vision or a Geiger counter or another device. The device interacts with the electron sufficiently to determine which slit, so the electron collapses down to a particle and goes through only one slit.
Once past the barrier, the electron, freed from interaction, reverts to its wavy state. Upon interaction with the detection screen, it again collapses down to a particle and lands as a tiny localized dot. Again, the video incorrectly shows that after repeated runs of the experiment, random dots cover the detection screen with no particular pattern. Experimental results show that the resulting pattern on the detection screen is, instead, two clumps as shown in Figure (1).
Consciousness and Wave Function Collapse
The role of consciousness in the collapse of the wave function has had a controversial history. In the early days of development of quantum mechanics, many of the founding fathers of the field contemplated the possibility that consciousness played a role in collapsing the wave function. Over time, this view was rejected. Considerable work was done starting in the 1950’s on the theory of decoherence. This is the theory that interaction with macroscopic particles cause collapse of the wave function. While this is a well-accepted theory, experiments in recent years also point to the possibility that consciousness can also cause collapse of the wave function. For further discussion, see the article in this encyclopedia collapse of the wave function.
In Summary
In summary, the electron is definitely a particle when it hits the detection screen. And at other times, it’s a wave. But it’s not a physical wave like a water wave or sound wave. It’s a wave that follows the laws of quantum mechanics.
