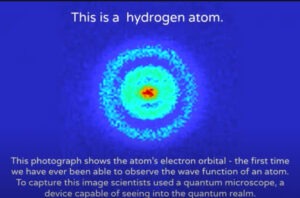

The image is a leap forward and exciting, but here are some other things the image is not: It’s not a photograph; it’s not of one electron; and it’s not of the electron wave function (which is an equation, not a material object). It should be noted that the scientists who created this image made none of these claims. It’s just that the image has sometimes been mislabeled by others.
Electron Orbital/Electron Cloud
Here is what the image shows: the region within a hydrogen atom in which the electron will most likely be detected. Red means “most likely,” and blue means “least likely.” The entire colored region is called an “orbital” or “electron cloud.”
Quantum physics tells us that an electron does not occupy a particular position until detected. This image of an orbital was reconstructed by computer based on detections of innumerable electrons.
Here is how the image was created: The experimenters in a Dutch lab zapped hydrogen atoms with a laser to energize the electron within the atom. This caused the electron to fly out of the atom and hit a detector half a meter away. The experimenters did this hundreds of thousands of times, each time with a new hydrogen atom. Depending on the frequency with which electrons hit different positions on the detector, the computer reconstructed an orbital, the region within the atom that the electron can occupy and where it might be detected. The New Scientist article provides more details.
Electron orbitals vary according to energy level.
The Dutch lab which created this image created additional images of electron orbitals in hydrogen atoms (below). Each image depicts an electron orbital at a different energy level, with (a) being the lowest and (d) the highest energy level. A low-energy electron orbital can be energized into a higher energy level with a laser. The laser emits innumerable photons. If the electron absorbs a photon, its energy level jumps up. Later, it may spit out the photon and settle back down to a lower energy level.

Prior to creation of these images, physicists calculated the shapes of orbitals based on quantum theory. They generated drawings like those below, sometimes using computers, based on the calculations.

