Spin is a property of subatomic and atomic particles. While spin was originally thought of as a particle twirling on its axis like a toy top, this interpretation was soon considered inaccurate. Nevertheless, people often continue to speak of spin as if it were a kind of rotating motion. This is because the experimental results of particle spin, in certain ways, parallel the twirling of a classical object. (A classical object is one that follows classical, that is, Newtonian laws, rather than quantum laws.)
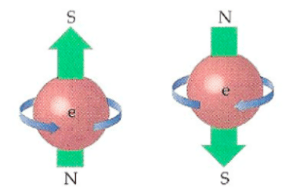
Physicists have not yet developed a visualization of physical reality that underlies spin. But they are able to describe spin mathematically and predict its behavior in lab experiments. Really, what they are predicting, however, is the magnetic properties of quantum particles, not their rotational motion. The key to understanding spin is to realize that, whatever it “really” is, its physical manifestation is magnetism.
Which Quantum Particles Have Spin?
Most fundamental subatomic particles, including electrons, have spin. The Higgs Boson is an exception—it does not have the property of spin. Composite particles, that is, protons, neutrons, the nuclei of atoms, and atoms, themselves, also have spin. However, to simplify a bit, this article focuses largely on electron spin.
Problems with the Spinning Top Analogy
When physicists calculated how rapidly electrons would have to spin to generate the strength of their magnetism, they would be spinning faster than the speed of light. Of course, this is a big problem because, according to Einstein’s Theory of Special Relativity, nothing can travel faster than the speed of light.
Calculations of spin also yield electrons larger than the size of the entire atom–another big problem. Electrons are actually a minute part of atoms. In fact, many physicists think of electrons as infinitesimal points, called “point particles.” Point particles have no extension in space at all, no volume—they’re conceived of as dimensionless points, like an ideal point in geometry. On top of everything else, how can a point particle have an axis about which it spins? This seems a self-contradictory concept.
Electron Spin and Magnetism
Electron spin has important effects in our everyday world. This is because electron spin is a major source of magnetism.

Axes of Electron Spin
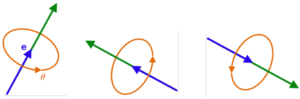
An electron can spin around an axis that points in any direction. Here’s what I mean: Imagine an arrow stuck through the center of an orange. The arrow could point any which way. The arrow would be the orange’s axis of spin. Similarly, the axis of spin of an electron can point any which way, and the electron spins around it.
The accompanying diagram shows three of the many directions in which the axis of a quantum particle could point. The number of directions includes 360° in each of the three dimensions. So, to say the axis can point in “many directions” is an understatement. In fact, there are an infinite number of possible directions for the axis.
Continuing with the orange, it could spin clockwise or counterclockwise around the arrow. The same is true of an electron: it can spin clockwise or counterclockwise around its axis.
Prior to measurement, the axis of the electron is in a superposition. That is, unless specially prepared, all one can say about the axis is the probabilities of it pointing in any particular direction. In addition, it is in a superposition of spinning clockwise and counterclockwise. Its spin and its direction of axis are assigned probabilities; but the direction of its axis and whether the spin is clockwise or counterclockwise are not defined until measured.
Measuring Electron Spin with a Stern-Gerlach Device
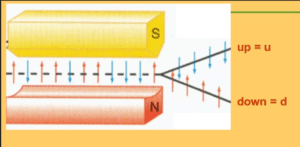
Here’s where quantum weirdness pops up: upon measurement, the axis of spin of an electron is always found to be the axis along which it is measured. The equations of quantum mechanics predict this result, and the experimental evidence supports it. In experiments, the spin can be measured by a Stern-Gerlach device.
If the Stern-Gerlach machine is set so that it measures spin only around a horizontal axis, each electron passing through the device will be found to spin around its horizontal axis, either clockwise or counterclockwise. Unless specially prepared, about 50% of the electrons will be found to have clockwise spin around the horizontal. About 50% will be found to have counterclockwise spin around the horizontal.
If the device, instead, is set to measure spin around the vertical axis, each electron will be found to spin around the vertical. Again, about half will spin clockwise and half, counterclockwise. The same will be found when measuring spin around an axis pointing in any direction: the axis of spin of the electrons will be found to be in the same direction. Half will spin counterclockwise and half counterclockwise.
Were the particles to behave like classical particles, their axes of spin would be found to point every which way. Instead, their axes are found to point only in the direction measured and their spin (up or down) is random around this axis. The spin of electrons is considered to be quantized, that is, upon measurement, it takes on only two values, up and down.
Stern-Gerlach Device, Read More

The accompanying video purports to show what would happen if little magnets, let’s say refrigerator magnets, were sent through the Stern-Gerlach device. They wouldn’t segregate themselves into an up group and down group. Instead, their north poles would be oriented every which way, and they would spread out into no particular grouping.
But this is more metaphor than reality. The Stern-Gerlach device wouldn’t actually work with little magnets.* But the intention is to get the idea across that if electrons obeyed classical laws, their axes of spin would be every which way. This would result in their spreading out evenly on the detector screen, not clumping together in the up position and the down position.
The video is accurate when it comes to showing what happens when electrically-charged quantum particles are sent through the Stern-Gerlach device, namely, they segregate themselves into an up group and down group.
As a final note about the video, it depicts the quantum particles, upon entrance to the device, as fuzzy balls. This fuzziness is to indicate that they are in a quantum superposition state. Later, they appear as solid blue and while spheres, indicating that upon detection, they have become physical particles with a definite axis of spin and direction of spin around the axis.
Origin of terms “spin up” and “spin down.” The terms “up” and “down” derive from the analogy with a spinning classical object like a top. When a toy top spins counterclockwise around a vertical axis, in classical physics, it is considered to have spin up. When it spins clockwise, it’s considered to have spin down. See the diagram above of electrons, which have been drawn as if they were like toy tops.
Relating counterclockwise spin to “up” may seem odd. Here’s the idea. Make a fist with your right hand and put it, thumb up, on a table. Your fingers will curl counterclockwise and your thumb points up. That’s the relationship between counterclockwise and up.
Now, turn your right hand so that your thumb is sticking down into the table. Your fingers are curling clockwise. So, clockwise fits with down.
As physicists don’t believe that electrons actually spin on their axes, the best way to interpret “spin up” is that it’s simply the opposite of “spin down.” However, if the spin of the particle were identified by using of a Stern-Gerlach device, more could be said. If the northerly magnet were the higher and stronger one, the spin up particles would be in the upper clump.
Spin Follows the Heisenberg Uncertainty Principle
As noted just above, an electron can have spin up or down along each of three axes. However, in accordance with the Heisenberg Uncertainty Principle (HUP), defining spin along one axis precludes defining spin along the other two. For example, let’s say that we measure the spin along Axis X as up. Then, we measure the spin along Axis Y as down. Here’s the problem: now that we know spin along Axis Y, the electron no longer has a specific spin along Axis X. Whatever we originally had measured along Axis X, we can now no longer rely as being accurate.
According to the Heisenberg Uncertainty Principle, the electron hasn’t even settled for itself whether its spin along Axis X is still up rather than down. Upon measuring the spin of Axis Y, the spins on Axis X and Z are not defined even by Nature, herself.
This same “logic” applies regardless of the axis. Definition of up/down spin on one axis precludes definition of spin on the other two axes.
Electron Spin ½
For an electron, the mathematical value assigned to spin up is +1/2 and to spin down, -1/2. You might ask, why ½? Good question, but the answer can be given only with advanced mathematics. The most I can say about this is that the mathematics makes it look, in some way, like the electron must cycle twice to get back to its original state. So, one spin around is only one-half a complete cycle. If it were to have Spin 1, cycling around once would return the particle to its original state. Again, this aspect of spin suggests actual rotational motion even though physicists reject it as a physical possibility.
Another description of what’s going on is based on imagining walking around the electron and being able to see it—no chance of that! But, let’s say you could. You would have to walk around twice (720°) to see the electron in the same orientation as when you started walking. This video shows a motion of a classical object which requires two revolutions to arrive back at its starting point: https://youtu.be/CYBqIRM8GiY. However, this should be seen as an analogy; assigning physical reality to electrons is problematic.
[See animation:Spin one-half slow animation. Youtube says that it’s an image, but it’s actually an animation. Was not able to add it to this page.]
Spin 1, Spin ½, Spin 0, and Possibly Even Spin 2
Different types of fundamental particles have been assigned different spin numbers: 0, ½, 1, 2…. Note, that these are assigned only to fundamental particles. The spin of composite particles like protons and neutrons is more complex.
As physicists don’t know the physical meaning of spin, the spin numbers are most accurately described at this time (2017) as simply just that: numbers. They are numbers that fit the equations that have been found to describe the behavior of particles in lab experiments.
All force-carrying subatomic particles (bosons) have Spin 1. This includes photons (which carry electromagnetic force), gluons (which carry Strong Force), and W and Z bosons (which carry Weak Force).
Matter particles (fermions), including electrons and quarks, have Spin ½.
The Higgs Boson is believed not to spin. It has Spin 0.
Physicists theorize that if gravitons exist, they would have Spin 2. Gravitons are hypothesized to carry the force of gravity but have not been detected as of yet (2017).
| Spin Numbers by Particle Type | ||
| Type of Particle | Examples | Spin Number |
| Bosons (force-carrying) | photon, gluon, W & Z boson | 1 |
| Fermions (matter) | electron, quark, etc. | ½ |
| Higgs Boson | 0 (no spin) | |
| Graviton (hypothetical particle) | 2 | |
Other Names for Spin
Spin is not due to an external force which sets a particle spinning. Nor does it appear due to actual physical motion of the particle. Particles seem to be “born” with spin as an inherent property like the mass or negative electric charge of an electron. Thus, spin is sometimes called “inherent” or “intrinsic” angular momentum.
Footnotes
