(Also called a “Stern-Gerlach machine.”) The Stern-Gerlach (SG) device detects the “quantum spin” of atoms and subatomic particles. Quantum spin is a property responsible for magnetism in atoms and subatomic particles. Using
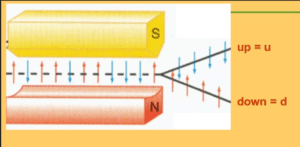
The video and diagram below explain the principles behind the experiment. The video is more metaphorical than realistic, but it gives a helpful overview. (See “Notes on the Video” at the end of this article for a necessary correction.) After viewing the video, I suggest looking at the diagram below and its explanation.
Original Stern-Gerlach Experiment
The device was named the “Stern-Gerlach device” (SG), after its German inventors, Otto Stern and Walter Gerlach. Their famous experiment of 1922 has since been repeated many times with a variety of quantum particles.
In the original experiment, Stern and Gerlach used silver atoms. These atoms have a magnetic north and south pole and generate a magnetic field around themselves. In the experiment the atoms travel through a magnetic field that varies in strength in different locations. The difference in strength is due to the top magnet having a point. This creates a stronger magnetic field than does the bottom magnet.* The silver atoms are deflected up or down depending on the orientation of their magnetic poles.
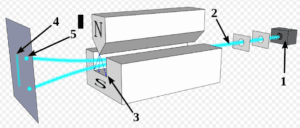
1: Furnace generates a beam of silver atoms. Each atom has a north and south magnetic pole. Were they classical objects, the atoms would have their north poles randomly oriented in space.
2: Beam of silver atoms (blue).
3: Magnetic field that is stronger and weaker in certain locations due to the north pole generating a stronger magnetic field than does the south pole. Of course, all magnets have both north and south poles, but the SG device is constructed to bring only a single north and single south pole in close proximity as shown.
4: Expected result–continuous vertical line of silver atoms hitting detection screen. This would occur if the north pole of each atom were completely randomly positioned in space. If the north pole were pointing straight up, the atom would be strongly deflected by the strong top magnet. As a result, the atom would be repelled towards the bottom of the detection screen. If, on the other hand, the south pole were pointing straight up, the atom would be attracted to the top magnet and would land at the top of the detection screen. Of course, one would expect most of the atoms to have poles oriented somewhere in between, in less extreme positions, and so, land anywhere between the top and bottom of the detection screen. As they landed, they would form a continuous vertical line on the detection screen.
5: What was actually observed–atoms hit the detection screen in only two spots. Those clumping at the upper spot are said, as explained below, to have “spin up.” Those clumping at the lower spot are said to have “spin down.”
Conclusion: The magnetism of silver atoms has only two states–north pole straight up or south pole straight up. The poles of the atoms do not have a continuous range of orientations as might be expected. The atoms are said to have “quantized spin.”
Magnetic Poles and Spin
The property of atoms and subatomic particles which generates their magnetism is called “spin.” This is due to an erroneous assumption that originally underlay the Stern-Gerlach experiment. This assumption was that the generation of a magnetic field by silver atoms is due to Maxwell’s Laws of Electromagnetism.
According to Maxwell’s laws, an object with electrical charge that spins develops a north and south magnetic pole and a magnetic field around itself. Physicists believed that a single electron in the outermost part of the silver atom was spinning and, thus, creating a magnetic field. However, they soon realized that this could not be the explanation for the magnetic behavior of the silver atoms.
When physicists calculated the speed necessary to create the strength of the magnetic field in the silver atoms, they found that electrons would have to be spinning faster than the speed of light—not possible. In addition, each electron would have to be as large as the silver atom, itself.
However, the name “spin” stuck. It remains the name of the magnetic property of electrons and other quantum particles. This is due, at least in part, to physicists finding other indications that point towards the visualization of spinning. For details of one such indication, see the second paragraph on the rotational inertia of particles in the Notes on the Video.
Stern-Gerlach Device—Notes on the Video
As a bit of trivia, while this video shows the pointed magnet at the top of the device being the south pole, in the original Stern-Gerlach experiment, it was the north pole. This is not a problem in the video – very possibly, SG experiments have been done since with a pointed top magnet being the south pole.
However, another aspect of the video is more metaphorical than realistic. The video starts by showing ordinary, tiny classical magnets streaming through the machine. In reality, tiny classical magnets are not placed in a Stern-Gerlach device because it wouldn’t be able to measure the orientation of their north poles. The stronger pointed magnet (the south magnet in the video version) would twist each tiny magnet so that after traveling through the device, every one of them would have their north poles pointing up. In other words, little classical magnets, after traveling through a Stern-Gerlach device, would not display the range of pole orientations shown in this video but, instead, would land in one spot.
In contrast to little classical magnets, a quantum particle seems to have inertia which resists such twisting. This inertia makes it similar to a little spinning gyroscope. Rotational inertia would explain why quantum particles, apparently, retain the orientation of their magnetic poles as they travel through the SG device rather than being twisted into a single orientation by the stronger pointed magnet. This behavior, again, points to the idea that the particle is physically spinning, despite physicists’ calculations showing that this would violate Special Relativity (namely, the speed of light).
The Transactional Interpretation of quantum mechanics proposes that quantum particles operate in a sublevel of reality. Familiarity with this interpretation may be helpful in visualizing what the particle is really doing.
Footnote
*This diagram shows the north pole of the magnet as pointed (creating stronger magnetism) and at the top of the device. This reflects its placement in the original Stern-Gerlach experiment. However, the device can, instead, be configured with a pointed south pole. And, so long as the two poles are facing each other, it doesn’t matter which is on top and whether they’re vertical, horizontal, or at some other angle relative to the floor. In the video accompanying this article, the south pole of a magnet is shown as pointed and on top.

 Click on the image for a video of the Stern-Gerlach device. See “Notes on the Video” at the end of this article for a necessary correction.
Click on the image for a video of the Stern-Gerlach device. See “Notes on the Video” at the end of this article for a necessary correction.