An atom is the tiniest component of an element which shares in the element’s properties. Break down matter any further and you’ve got electrons, protons, and neutrons. But these components, electrons, for example, do not share in the element’s properties.
Au100.JPG Retrieved June 17, 2017]
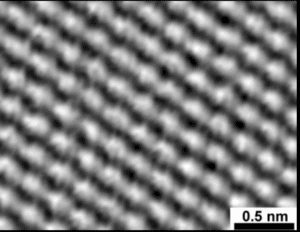
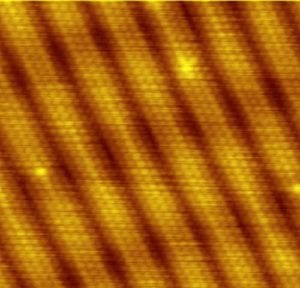
Gold atoms. In gold, the atoms at the surface arrange themselves in diagonal columns. Each column is several atoms across. Dark channels form between the columns.
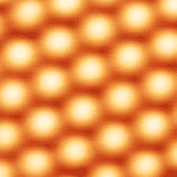
Carbon atoms. Carbon atoms arrange themselves into a few forms including diamonds and graphite. When the atoms are arranged in one pattern, they form diamonds. When arranged in another, they form graphite. Both diamonds and graphite are crystals. The arrangement shown in the accompanying image is the graphite arrangement of carbon atoms. Graphite is used in making pencil leads among other things.
Atoms of Solids
Each atom appears as a little spherical cell. While it’s not possible to capture in a still image, all atoms, whether in a solid, liquid, or gas, jiggle about rapidly. They vibrate.
How these images of atoms were made. These images of atoms were created by a high-powered microscope called a “scanning tunneling microscope.” An ordinary microscope, which relies on light to form an image, would not be sufficiently powerful to allow us to see atoms. Instead a scanning tunneling microscope uses a stylus with a tip made of a single atom to probe or “feel” materials, atom by atom. The electronic display of the microscope creates an image that is formed by what the probe has felt.
Atoms of Liquids
However, the fundamental components of most liquids are not atoms, but molecules. For example, liquid water is made of molecules formed by the bonding of two hydrogen atoms with one oxygen atom. (See a diagram of a water molecule lower on the page.) Liquid helium, which liquefies at a temperature near absolute zero, is an example of a liquid formed by atoms rather than molecules. It took me a while to find an example of a liquid formed by atoms.
Atoms of Gases
However, most gases, like most liquids, are composed of molecules rather than atoms. Even pure oxygen gas, an element, is formed by two oxygen atoms bonded together to form a molecule. Thus, oxygen gas is referred to as O2, referring to two atoms. This is the form that oxygen takes in air which we breathe.
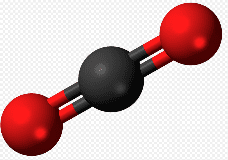
Carbon dioxide, another major component of the air we breathe, is formed by a carbon atom bonded with two oxygen atoms. The symbol for carbon dioxide is CO2. A diagram of a carbon dioxide model appears lower on this page.
Components of Atoms–Subatomic Particles
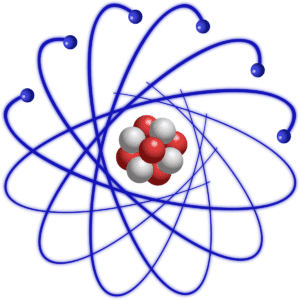
Atoms are made of a nucleus surrounded by an electron cloud.

The video shows some of the subatomic particles that make up an atom. The text underneath the video explains that it’s showing a nucleus of protons and neutrons surrounded by electrons. All atoms take this form. Even though an atom of carbon, let’s say, is quite different from an atom of gold, they’re both made of the same components: a nucleus of protons and neutrons surrounded by electrons.
The differences between carbon and gold (or any other element) are due to having differing numbers of protons. While the video says that the different elements have differing numbers of electrons, it’s really the number of protons that define each element. In fairness to the video, usually, though not always, an atom has the same number of protons as electrons.
If you would like to know the number of protons in each element, see the Periodic Table of Elements. Each element has one more proton in its nucleus than in that of the prior element on the table.
Examples of Atoms, Showing Their Components

[Image source: Commons:Robert Lavinsky https://commons.wikimedia.org/wiki/File:Graphite-and-diamond-with-scale.jpg]
they’re vibrating energy. Similarly, electrons aren’t little balls that
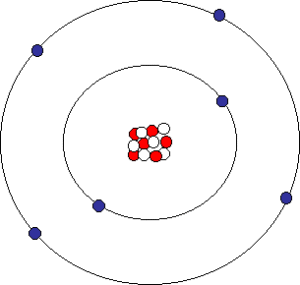
Oxygen atom. As shown in the accompanying diagram, oxygen has 6 protons (red) and 6 neutrons (white) in the nucleus, surrounded by 6 electrons.
Atoms Have a Neutral Electrical Charge
The nucleus of the atom is composed of neutrons and protons. Neutrons have no electrical charge, but each proton has a single positive electrical charge. The protons in the nucleus give the nucleus an overall positive charge.
Each electron, on the other hand, has a single negative electrical charge. Commonly, within the atom, each proton in the nucleus is balanced by an electron vibrating around it. The positive and negative charges balance each other, canceling each other and yielding an atom that is electrically neutral. For this reason, we can touch matter made of atoms without continually receiving electrical shocks.
Difference Between Atoms and Molecules
As mentioned, most matter that we deal with in daily life is made up of atoms joined together in molecules rather than individual atoms. The atoms of the element, carbon, for example, join with atoms of the element, oxygen, to form molecules of carbon dioxide. Carbon is a black solid, and oxygen is a colorless gas that our bodies breathe. They join to form carbon dioxide, a colorless gas that, if breathed in excessive quantities, can poison us.
As this example demonstrates, when atoms of elements bond with atoms of other elements to create molecules, the molecules take on properties that often bear little or no resemblance to those of the original atoms.
Metals not composed of molecules. Metals, however, are a special case–many metals are not composed of molecules. As shown in the image of gold atoms at the beginning of this article, the atoms arrange themselves in an organized structure, called a “lattice.” The nuclei of the atoms are held together in this strong lattice structure. But, the electrons flow about freely, not bound to individual atoms. Each nucleus carries positive electrical charge due to the positive charge of protons within it. The electrons are negatively charged. Opposite charges attract, and the sharing of electrons bind the positive nuclei together.
Ions. It’s more accurate to call the gold nuclei in the metal lattice “ions” rather than “atoms.” An atom is a nucleus with a full complement of electrons so that it has no overall electrical charge. An ion is an atomic nucleus that carries an electrical charge. An ion is positively charged if it has a deficit of electrons and negatively charged if it has a surplus of electrons. Rather than individual atoms or molecules, the metal lattice is formed by positive ions in a sea of electrons.
Pieces of metal which take this lattice form are crystals. This is the usual form that metals take. If, due to special conditions, the lattice form is not taken, the metal does contain atoms and is said to be in the “amorphous form.”
If we were to touch the lattice form of gold with an electric current, the free-flowing electrons would conduct the electricity easily. While gold is an excellent conductor of electricity, it would be too expensive to use in electrical wires.
Gold and other metals which are formed by ions are good conductors of heat as well as electricity. In the case of a few such metals, like iron, the free-flowing electrons also render the metal magnetic.
How big are atoms?
An atom is 1/100,000 the width of a human hair, a human hair being one of the smallest things that we can see with our bare eyes. In other words, if we could place 100,000 atoms side-by-side, they would span the width of a human hair. Another way to describe the size of an atom is that there are more atoms in a glass of water than there are glasses of water in the oceans of Earth.
While all atoms are tiny, they do vary in size. The atom with the biggest radius, cesium, is nine times wider than the atom with the smallest radius, helium.
Atoms are too small to see with ordinary microscopes that work by relying on visible light. However, images of atoms can be created using scanning tunneling microscopes, a kind of electron microscope, as described in the section above “How the images of atoms are made.”
Isotopes
Atoms with the same number of protons but differing numbers of neutrons are isotopes of each other. For example, Uranium 238 and Uranium 235 are isotopes of the element uranium. They both have 92 protons. But Uranium 238 has three more neutrons, as signified by the number 238. For more information, see the entry for “isotope.”
Atoms as Embodied Mathematical Equations
Are all atoms identical if they are of the same element? This question has a subtle answer. On the one hand, we can say that atoms are made of the same components (protons, neutrons, and electrons). And every proton is identical to every other proton; every neutron is identical to every other neutron; and every electron is identical to every other electron. Since atoms of the same element have the same number of each of these components, we might think that atoms of the same element are identical.
On the other hand, two atoms of the same element can differ because: 1) They might be different isotopes, that is, have different numbers of neutrons in their nucleus, 2) They might be different ions, that is, might have different numbers of electrons, and 3) Their component subatomic particles might be in different quantum states. An example of a different quantum state would be an electron that has higher energy than another electron.
For these reasons, two atoms of the same element might be measured as different from each other. However, each atom has exactly the same potential to be like any other atom. All atoms of an element have exactly the same potential to become a particular isotope, a particular ion, or to take on a particular quantum state.
Atoms and the subatomic particles within them can be completely described by mathematical equations. It’s not necessary to provide samples of a particular atom. It’s fully describable by the equation for every atom of the same type, that is, of the same element (allowing for differences due to the three factors listed above).
Frank Wilczek, a Nobel-prize-winning quantum physicist, makes this point in regards to the subatomic particles within protons, namely, quarks and gluons:
“The point I want to emphasize here…is that quarks and gluons…are mathematically complete and perfect objects. You can describe their properties completely using concepts alone, without having to supply samples or make any measurements….Gluons are the objects that obey the equations of gluons. The its are the bits.”
While Wilczek is writing about subatomic particles that form protons, the same can also be said of atoms—they are completely describable by mathematical equations. They are embodied mathematical information. They can be fully and completely described by information, by bits. Quarks and gluons, are “its,” that is particles. They arise from information, that is, “bits.” John Wheeler, an important quantum physicist, originally coined Wilczek’s phrase as “its from bits.”
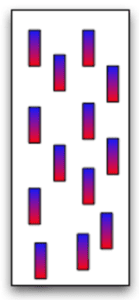
How do atoms form magnetic materials?

Imagine the free-flowing electrons of a metal. Each electron has a north pole (blue) and a south pole (red). In the image to the left, the electrons point every which way so the metal is not magnetic.
Etymology of “Atom”
The ancient Greeks, notably the philosopher, Democritus (about 460 BC to 370 BC), proposed that matter is made of tiny individual particles. The Greeks called these particles atomos. The word meant “cannot be divided.” Democritus believed that a small number of different types of atoms were arranged in a variety of patterns to form all the matter that we see around us.
After more than 2,000 years, scientists have, finally, been able to confirm the basic notion of Democritus that all matter is made of a small number of different types of atoms, the different atoms of the 100 or so elements.
However, today, we know that atoms, themselves, can be further divided into even smaller particles. So, the ancient Greek name atomos “cannot be divided” is not as applicable as Democritus had hoped.
