In 1913, the physicist, Niels Bohr, proposed this new model of the atom. Even though it’s now considered obsolete, at the time it was a huge step forward in quantum physics. Bohr built it on the model that his mentor, Sir Ernest Rutherford, had proposed. To appreciate the Bohr model of the atom, it’s necessary to understand the earlier model, the Rutherford model.
Rutherford Atom
In the Rutherford model, electrons orbit the nucleus like planets around the sun. Rutherford and other physicists knew at the time that the “solar system
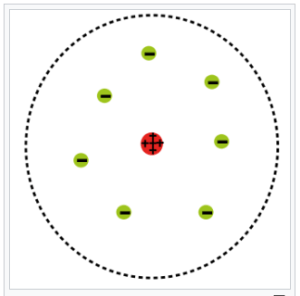
Physicists were well aware of its fatal flaw: According to Maxwell’s laws of electromagnetism, the electrons would radiate electromagnetic energy as they circled the nucleus. They would, in this way, lose energy extremely quickly. As the electron is negatively charged, without sufficient energy to keep it in orbit, it would become vulnerable to the attraction of the positively-charged protons in the nucleus. As a result, it would almost immediately fall into the nucleus. This contrasts with what happens in reality—electrons don’t fall into the nucleus. Thus, matter sits all around us, very stably.
Bohr Atom
Bohr improved on the Rutherford model by proposing a new law that applies only to the quantum (subatomic and atomic) world. He proposed that Nature allows electrons to occupy only specific orbits. The distance of each orbit from the nucleus corresponds to the electron’s energy level. The greater the energy, the greater the distance from the nucleus.
Quantum Leaps
According to this law, electrons do not lose energy as they circle the nucleus but are constrained within whichever orbit they happen to occupy. Only when they are fed energy, as when they absorb a photon, can they jump to an orbit more distant from the nucleus. This is the famous “quantum leap.” The mathematical equations indicate that the jump from one orbit to another is instantaneous; it does not involve crossing the distance in between. The same is true when an electron falls to a closer-in, lower-energy orbit—it must spit out energy, for example in the form of a photon. Again, it does not travel across the distance to the closer-in orbit; it makes an instantaneous quantum leap. First it is in one orbit; the next moment it’s in another.
By this law of Nature, electrons are kept from spiraling into the nucleus. Bohr’s model of the atom was one of the first steps in the realization that the quantum world operates on different laws from the everyday world that we live in. For this work and his other important contributions to the founding of quantum physics, Bohr was awarded the Nobel Prize in 1922.
Upgrading the Bohr Atom
Bohr’s model has severe limitations. For one thing, it works only for the hydrogen atom. When an atom has two or more electrons, calculations based on the Bohr model do not match experimental results.
But even in the case of hydrogen, physicists were uncomfortable with the “law of nature” that prevented the electron from falling into the nucleus. What was the mechanism of this law? One book on quantum mechanics notes, “By fiat, Bohr’s formula ‘forbid’ an electron to crash into the nucleus.” Physicists at the time made just such comments about Bohr’s law of nature.
Later, it was found that the Heisenberg Uncertainty Principle provides the needed mechanism. According to the Heisenberg Uncertainty Principle, orbiting quantum particles resist being pinned down to a well-defined position. Sitting tightly up against the nucleus would closely define the position of the electron. As the electron approaches the nucleus, it experiences resistance and pushes away.

The Bohr atom was replaced in the 1920’s by the equations of quantum mechanics developed by Werner Heisenberg and Erwin Schrodinger. Quantum mechanics, itself, has since been upgraded and has become today’s Quantum Field Theory. The equations of Quantum Field Theory accurately describe experimental results for atoms of all the elements. For more information about the current view of the atom in Quantum Field Theory, see the article atom.
