Isotopes are a special kind of atom. Atoms with the same number of protons but different numbers of neutrons are isotopes of each other. For example, Uranium 235 and Uranium 238 are isotopes of the element uranium. They both have 92 protons in their nuclei but have differing numbers of neutrons. The nucleus of Uranium 235 has 92 protons plus 143 neutrons (92 + 243 = 235). The nucleus of Uranium 238 has 92 protons plus 146 neutrons (92 + 146 = 238).
Atom, Proton, and Neutron Briefly Defined
An atom is a tiny component of matter. An atom is 1/100,000 the width of a human hair, a human hair being one of the smallest things that we can see with our unaided eyes. In other words, if we could place 100,000 atoms side-by-side, they would span the width of a human hair, and we would barely be able to see the lot of them as a tiny dot.
An atom is composed of a nucleus surrounded by a negatively charged electron or electrons. Within the nucleus is at least one proton, a positively charged particle and, for the vast majority of elements, one or more neutrons. Protons and neutrons are similar in mass and about 1,837 times heavier than electrons.
An Example–Isotopes of the Hydrogen Atom
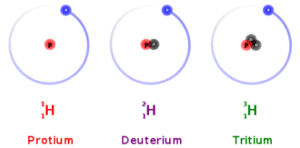
The hydrogen isotopes act similarly in chemical reactions. For example, all three combine with oxygen to form water molecules. In fact, the ocean is one of the best sources of deuterium and tritium. A small fraction of water molecules in the ocean include atoms of deuterium or tritium rather than ordinary hydrogen.
Isotopes Behave Similarly in Chemical Reactions
Isotopes behave similarly to other isotopes of the same element in chemical reactions because such reactions don’t depend on the properties of atomic nuclei. Rather they depend on the number and arrangement of an atom’s electrons.
Because isotopes react similarly in chemical reactions, it can be quite difficult to separate one isotope from another. This creates a challenge when separating isotopes that are radioactive from non-radioactive isotopes of the same element. Scientists have developed separation techniques that are used in both creating fuel for both nuclear bombs and nuclear reactors.
Isotopes Vary in Weight and Radioactivity

