The Periodic Table of the Elements lists all the elements, over 100 of them, in a specific sequence. This includes the elements that occur in nature plus those that scientists have created in laboratories. Elements are also called “chemical elements.”
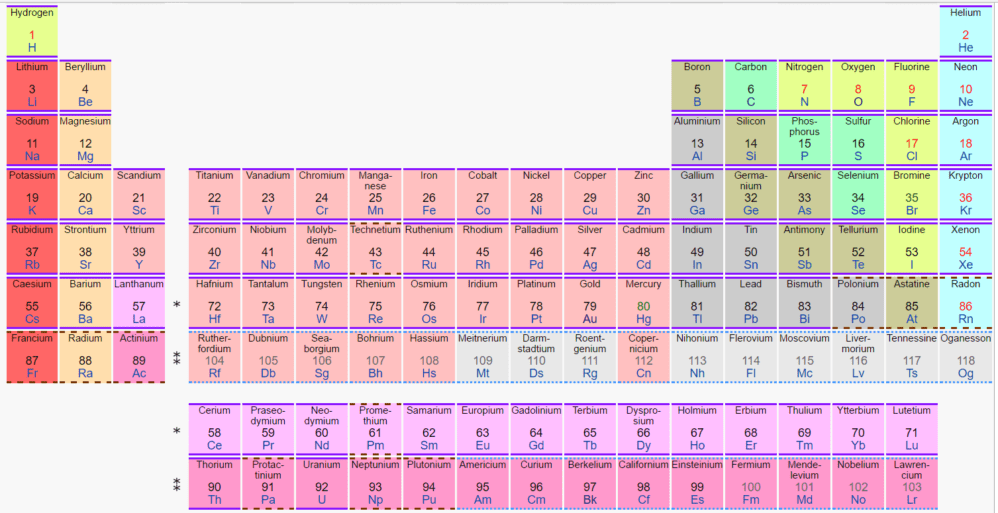
The number of protons, called the “atomic number,” is shown in the center of each square of the table. For example, the first element, hydrogen (upper left corner), has one proton and is assigned Atomic Number 1. Atomic numbers in this table run from 1 to 118. Later editions of the table may well list additional elements with even higher atomic numbers because scientists continue to create new ones in laboratories.
The first 94 elements occur in nature, with plutonium being Atomic Number 94. Elements with higher atomic numbers than 94 may also occur in tiny amounts in nature due to naturally occurring radioactive decay. But above plutonium, the elements in the Periodic Table are usually seen only in laboratories. These laboratory-created elements have large nuclei that are unstable and fall apart quickly. Of course, it’s also possible to create elements with atomic numbers lower than 94 in the laboratory.
The Periodic Table holds considerable information besides the atomic number of each element. The colors, symbols, and exact arrangements of the elements all have meanings which are well worth further study.
