To ionize is to convert a substance from atomic or molecular form to ions.
An ion is a type of atom that has an imbalance of electrical charge. Ordinarily, an atom has the same number of electrons as protons. Each electron has one
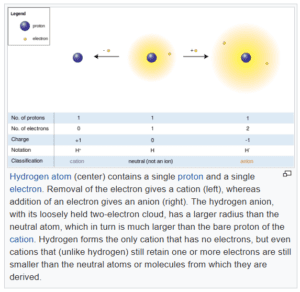
An ion may have an excess of electrons relative to protons, in which case, it has a negative charge. Or it may have a deficit of electrons relative to protons, in which case, it has a positive charge.
The accompanying diagram shows a hydrogen atom in the middle with a positive ion on the left. It has lost an electron, leaving it as an ion with a positive charge. On the right, the atom has gained an electron and so, is a negatively-charged ion.
For more information, see ion.
