The atomic number of an atom is the number of protons in its nucleus. For example, an atom of carbon has six protons and has Atomic Number 6. An atom of oxygen has eight protons and has Atomic Number 8.
The atomic number determines the chemical element. In other words, for the most part, the differences between the elements, carbon and oxygen, stem from the difference in the number of their protons, their atomic number.
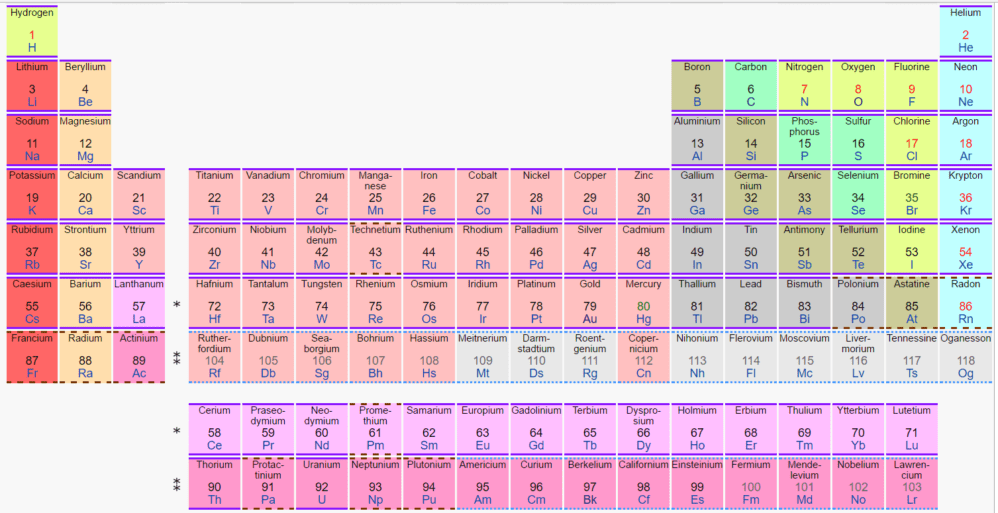
Atomic Number in the Periodic Table of the Elements
The Periodic Table of the Elements lists the elements in the sequence of their atomic number. In the copy of the table which appears in this article, the atomic number is at the center of each cell.
Difference Between Atomic Number and Atomic Mass
Atomic mass is different from atomic number. Atomic mass is the total mass of an atom’s protons, neutrons, and electrons.
